Try our favorite, clean protein powder: See our top pick →
Try our favorite, clean protein powder: See our top pick →
Evidence Based Research To fulfill our commitment to bringing our audience accurate and insightful content, our expert writers and medical reviewers rely on carefully curated research.
Read Our Editorial Policy
The term “walking dead” is most often associated with a popular zombie post-apocalyptic TV show, but it can also apply to a regular occurrence inside the human body.
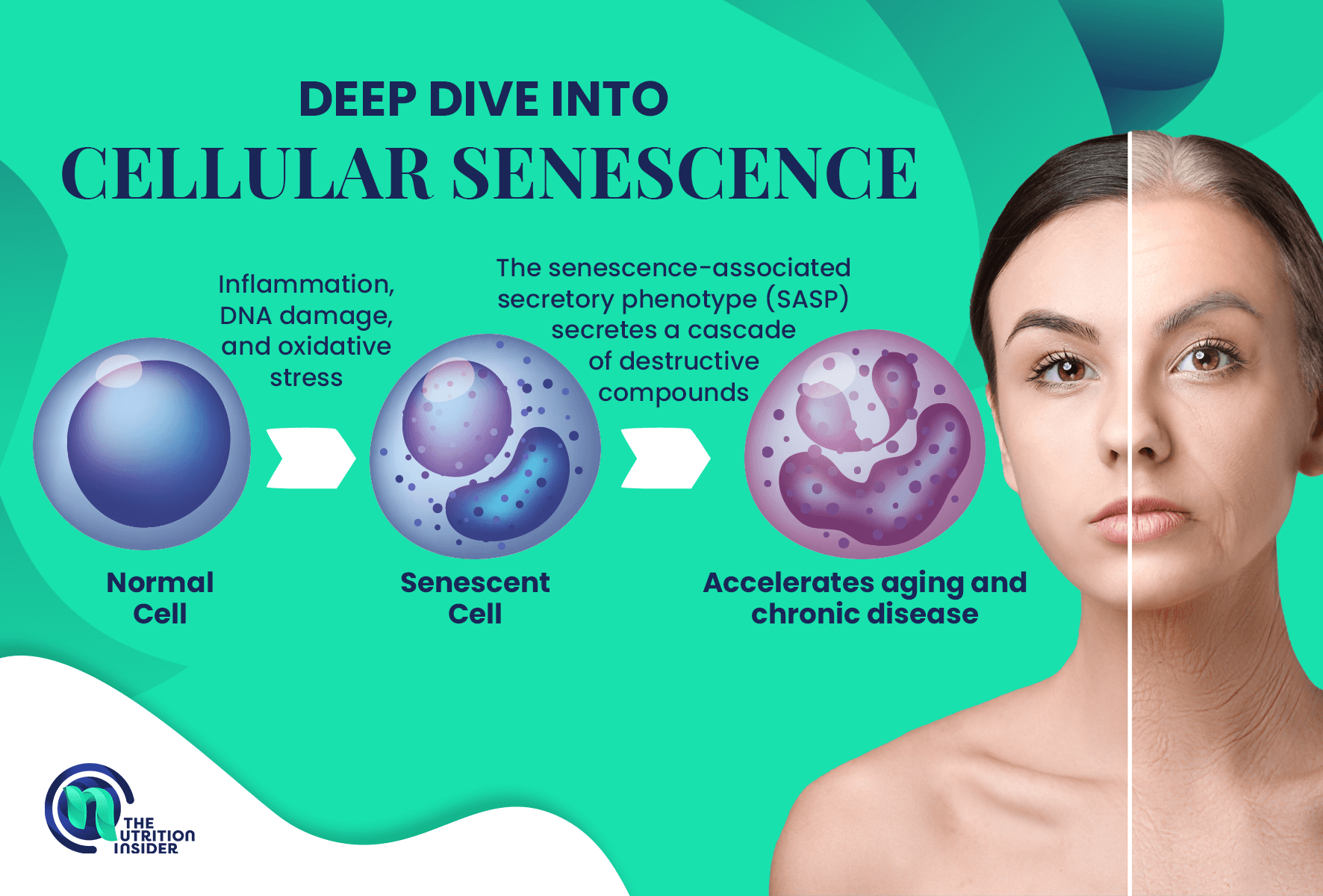
Also known as cellular senescence, these so-called “zombie cells” lose their ability to divide but remain in the body, unable to fully die.
These dysfunctional cells are linked to accelerated aging, chronic disease development, and the physiological decline of organs, systems, and tissues all over the body.
In this article, we’ll take a deep dive into what cellular senescence is, how it affects the body, and ways to slow down senescence to support healthier aging.
Simply put, senescence is when cells stop dividing and lose their function.
This cell cycle arrest occurs with increasing age or in response to various stressors, including inflammation and an accumulation of free radicals or reactive oxygen species—unstable compounds that cause oxidative stress, damaging cells and DNA.
Back in the 1960s, researchers Leonard Hayflick and Paul Moorhead discovered that normal human cells only replicate for a set number of times, after which they become senescent.
This process is now known as replicative senescence, or the Hayflick limit, which entails that human cells only replicate and divide between 40-60 times before they can no longer.
After reaching its Hayflick limit, a cell will either undergo apoptosis—programmed cell death—or become senescent.
However, unlike apoptosis, which kills a cell, senescent cells lose function but do not die, leaving a trail of inflammatory cellular debris in their wake.
Instead, senescent cells enter a half-alive state (hence the nickname “zombie cells”) that perpetuates damage to the rest of the body.
This damage occurs through the senescence-associated secretory phenotype (SASP), which secretes a cascade of destructive compounds, including cytokines, chemokines, and growth factors.
Like that one moldy berry that corrupts the whole carton, the destructive compounds from this senescent phenotype cause inflammatory damage to neighboring tissues and cells.
As a result, this is one way that cellular senescence is thought to contribute to age-related tissue and organ dysfunction and various chronic diseases.
But, it’s important to note that senescence isn’t always bad.
It can act as a tumor suppressive mechanism, limiting the replicative capacity of a new tumor cell to halt cancer development.
However, chronic senescence is also implicated in the development or progression of some cancers, giving it a paradoxical role.
Transient or short-lived senescence is also critical for wound healing and tissue regeneration after injury.
Overall, it’s the chronic and accumulated senescence that is detrimental to health and aging.
Another key player in the aging process are telomeres.
Telomeres can be imagined as the plastic casing protecting the tip of a shoelace, as telomeres are repetitive strands of DNA that “cap” and protect the ends of our chromosomes, where sensitive genetic information is stored.
Cellular senescence and telomeres go hand in hand, as telomeres shorten with every cell division.
When a cell reaches the end of its telomere, it can no longer divide—it’s reached its Hayflick limit—and will undergo either apoptosis or become senescent.
Like senescence, accelerated telomere shortening is linked to an increased incidence of disease development and aging.
Cellular senescence can affect every tissue and system in the body, including the immune system, skin, brain, adipose tissue, and other internal organs, like the liver, heart, and kidneys.
Although we might think that skin aging mainly comes from external sources like UV damage from one too many sunburns, a lot of aspects of unhealthy skin can arise from cellular senescence.
In the skin, senescence can be induced prematurely due to DNA damage or excessive oxidative stress, which leads to wrinkles, hyperpigmentation, thin or inflamed skin, and an increased risk of skin cancers.
Research has shown that several polyphenolic antioxidants found in many plants—including those from pomegranates, tomatoes, grapes, olive oil, chaga mushroom, and seaweed—exert protective effects against skin senescence.
Adipose (fat) tissue is the organ that can change most dramatically throughout life.
Fat cell precursors called preadipocytes can trigger pro-inflammatory signaling pathways that are highly susceptible to senescence (as are all progenitor cells, also known as stem cells.)
The inflammatory states of both senescence and obesity can exacerbate each other, leading to detrimental health consequences like metabolic dysfunction and chronic disease development.
Studies have found up to 30-fold more senescent cells in visceral adipose tissue—the harmful kind of fat that surrounds internal organs—in obese adults than non-obese adults.
Excessive cellular senescence in the brain contributes to Alzheimer’s disease and other neurodegenerative disorders.
Senescent cells have been detected in the brains of people with Alzheimer’s disease, and the removal of senescent cells in animal models improves memory in studies with aged mice.
Telomere shortening, neuroinflammation, and heightened oxidative stress are also thought to contribute to aging in the brain.
Senescence is thought to affect just about every organ in the body, including the kidneys, liver, heart, joints, and intestines.
For example, the accumulation of senescent cells is associated with an increased risk of heart disease, kidney disease, and osteoarthritis.
Senescence is one component of aging—but not the entirety of it.
Aging is a process of gradual physiological deterioration, while cellular senescence can happen at any age (although it is more common with increasing age).
Cellular senescence can begin at any life stage—even as early as embryonic development, where it is a beneficial process.
However, senescent cells accumulate more rapidly in older adulthood; around age 60, there tends to be an exponential increase over the following years.
Toward the end of life, up to 10% of tissues exhibit some markers of senescence, especially in unhealthy individuals.
No, senescent cells are resistant to the normal cell death called apoptosis.
Instead, senescence is associated with a “half-alive” state, where the cell is still living but is unable to perform its typical replicative roles.
A lot of recent research has focused on reducing senescent cells with senolytics—compounds, drugs, or chemicals that effectively kill and remove senescent cells from the body.
However, these zombie killers tend not to have specific targets, sometimes leading to accidental clearance of non-senescent cells and increasing the risk of toxic side effects.
One commonly used senolytic is a combination referred to as DQ—the chemotherapy drug dasatinib plus quercetin, an antioxidant compound found in many fruits and vegetables.
Many prefer to use senolytics made with natural ingredients, including Neurohacker’s Qualia Senolytic, which utilizes quercetin, fisetin, curcumin, and ginseng root extract to remove senescent cells from the body.
Products like these use intermittent, short-term supplementation dosages—as short as two days per month—to limit the possibility of side effects.
Research also indicates that regular exercise can reduce senescent cell burden.
On the preventative side, maintaining healthy habits throughout the entire lifespan—including eating a nutritious and anti-inflammatory diet, staying active, and not smoking—can help to ward off excessive cellular senescence with age.
As cellular senescence is an internal process, it can be difficult to ascertain specific signs or symptoms of aging to senescent cells.
Experiencing common age-related signs of physiological decline, like slower movements, wrinkled skin, aching joints, and vision or hearing loss, likely go hand in hand with increased senescence.
However, these symptoms cannot be attributed explicitly to senescent cells.
While senescent cells can certainly be removed from the body, it’s generally accepted that once a cell reaches the end of its telomere, the subsequent growth arrest is irreversible.
However, although this cell cycle arrest that occurs in senescence is thought to be permanent, recent research suggests that some senescent cells can re-enter the cell cycle—like cancer cells—or even be reprogrammed into induced pluripotent stem cells (cells that can reprogram to become any type of cell).
Amaya-Montoya M, Pérez-Londoño A, Guatibonza-García V, Vargas-Villanueva A, Mendivil CO. Cellular Senescence as a Therapeutic Target for Age-Related Diseases: A Review. Adv Ther. 2020;37(4):1407-1424. doi:10.1007/s12325-020-01287-0
Banito A, Gil J. Induced pluripotent stem cells and senescence: learning the biology to improve the technology. EMBO Rep. 2010;11(5):353-359. doi:10.1038/embor.2010.47
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578-582. doi:10.1038/s41586-018-0543-y
Csekes E, Račková L. Skin Aging, Cellular Senescence, and Natural Polyphenols. Int J Mol Sci. 2021;22(23):12641. Published 2021 Nov 23. doi:10.3390/ijms222312641
Justice JN, Gregory H, Tchkonia T, et al. Cellular Senescence Biomarker p16INK4a+ Cell Burden in Thigh Adipose is Associated With Poor Physical Function in Older Women. J Gerontol A Biol Sci Med Sci. 2018;73(7):939-945. doi:10.1093/gerona/glx134
Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. 2017;21:21-28. doi:10.1016/j.ebiom.2017.04.013
Liu RM. Aging, Cellular Senescence, and Alzheimer’s Disease. Int J Mol Sci. 2022;23(4):1989. Published 2022 Feb 11. doi:10.3390/ijms23041989
Liu J, Wang L, Wang Z, Liu JP. Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells. 2019;8(1):54. Published 2019 Jan 15. doi:10.3390/cells8010054
Schafer MJ, White TA, Evans G, et al. Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue. Diabetes. 2016;65(6):1606-1615. doi:10.2337/db15-0291
Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667-684. doi:10.1111/j.1474-9726.2010.00608.x
Subscribe now and never miss anything about the topics important to you and your health.